Indian-Made Alertness Drugs Trigger Severe Skin Reactions in Singapore
At least nine people in Singapore required hospitalization between February 2024 and February 2025. They developed serious skin conditions after consuming Modafinil and Armodafinil tablets. These drugs originated from India and users obtained them without doctor prescriptions.
Unregistered Drugs Sold Illegally in Geylang
Singapore's Health Sciences Authority issued a warning on Monday. The regulatory body stated the medicine is not registered in the country. Individuals procured the tablets in the Geylang neighborhood from street peddlers or friends. Their goal was to boost alertness and energy levels.
The HSA emphasized that Modafinil treats conditions like narcolepsy in some nations. Narcolepsy causes excessive daytime sleepiness. However, the drug remains unregistered for use in Singapore. The affected individuals ranged in age from 18 to 57 years old. This group included two women.
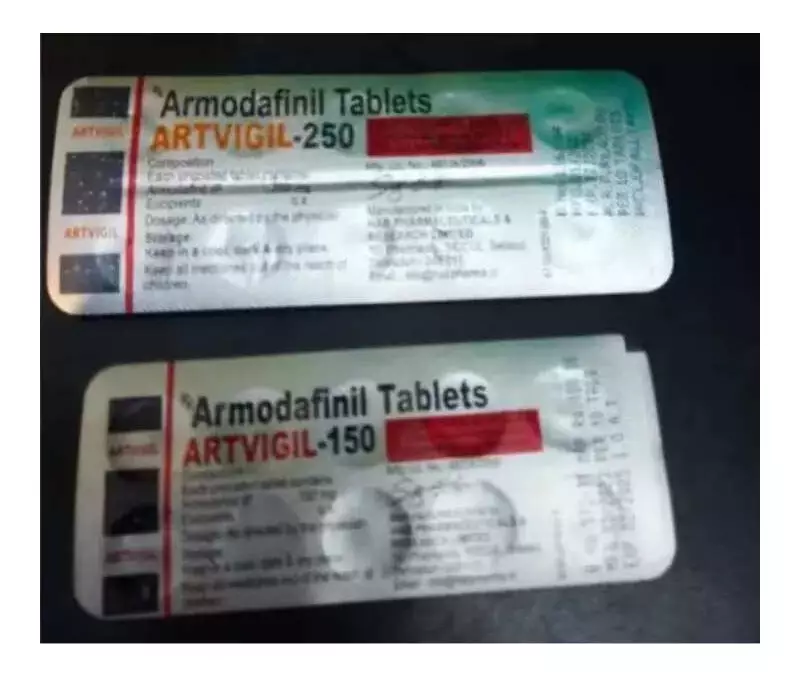
Tablets Traced to Dehradun Pharmaceutical Company
The HSA did not reveal the manufacturer's identity. However, investigations identified the tablets from pictures circulated in local Singaporean and Southeast Asian media. The drugs came from a pharmaceutical company located in Dehradun, Uttarakhand.
Drug strips showed a manufacturing date of 2023. The expiry date was listed as 2025. The HSA advisory clarified a critical point. Hospitalized patients used these medicines inappropriately to enhance energy. They purchased them from street vendors without any medical prescription.
Life-Threatening Skin Conditions Diagnosed
Six of the nine hospitalized patients developed Stevens-Johnson Syndrome. This is a life-threatening condition. It causes severe blistering and peeling of the skin and mucous membranes. Three patients suffered from toxic epidermal necrolysis. This represents an even more severe form of SJS.
Dehradun Administration Pledges Legal Action
Dehradun District Magistrate Savin Bansal addressed the situation. He stated the administration would take necessary legal action after reviewing details through government channels. Bansal affirmed the district administration's commitment to public health safety.
"We have already issued directions to concerned officials," Bansal said. "They must register a case and make necessary arrests in such incidents." The administration aims to prevent similar occurrences in the future.
This incident highlights the dangers of obtaining prescription drugs through unofficial channels. It underscores the importance of using medications only under proper medical supervision.





