In a remarkable achievement for India's healthcare sector, a domestically manufactured next-generation heart stent has earned prestigious international recognition, positioning the country as a serious player in advanced medical device manufacturing.
Global Validation for Indian Medical Innovation
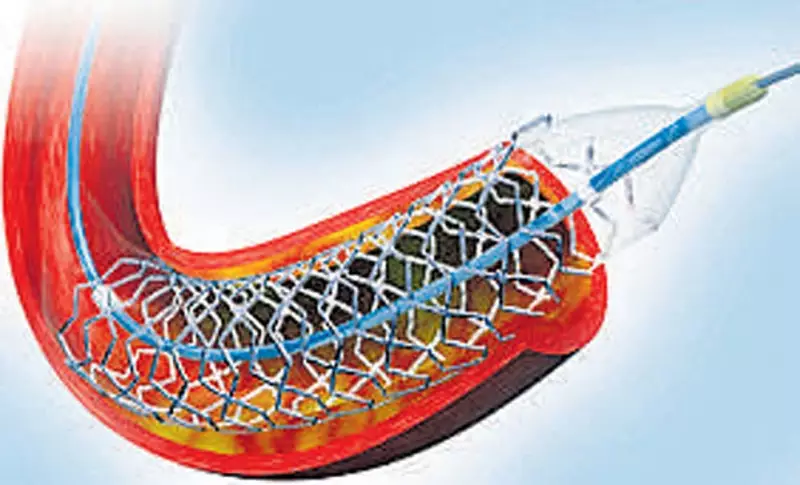
The Supraflex Cruz sirolimus-eluting stent, developed and manufactured by Sahajanand Medical Technologies in Surat, has received the CE Mark certification from European authorities. This certification represents a significant milestone, as it validates the stent's safety, performance, and quality according to stringent European Union medical device regulations.
Superior Technology for Better Patient Outcomes
What makes this stent particularly noteworthy is its advanced technological features. The Supraflex Cruz incorporates a unique flexible stent design combined with an ultra-thin strut structure. This combination allows for better deliverability through complex coronary anatomy and potentially improved clinical outcomes for patients with coronary artery disease.
The stent's innovative design includes:
- Enhanced flexibility for navigating challenging blood vessel pathways
- Ultra-thin struts that may reduce vessel injury and inflammation
- Advanced drug-eluting properties to prevent restenosis
- Proven efficacy in complex patient cases
Clinical Evidence Supporting Excellence
The international recognition follows compelling clinical evidence from multiple global studies. Research has demonstrated that the Supraflex Cruz stent performs comparably to, and in some aspects even surpasses, established international brands in terms of safety and efficacy.
This validation is particularly significant because it comes from independent international studies rather than manufacturer-sponsored research, adding credibility to the device's performance claims.
Impact on Global Healthcare Accessibility
This development represents more than just a technical achievement—it has substantial implications for global healthcare accessibility. Indian-manufactured medical devices typically come at a more affordable price point compared to their Western counterparts, potentially making advanced cardiac care more accessible to patients in developing countries.
The recognition also opens up new export opportunities for Indian medical device manufacturers in European and other international markets, strengthening India's position in the global medical technology landscape.
A Boost for Make in India Initiative
This achievement aligns perfectly with India's broader ambitions to become a global hub for medical device manufacturing. It demonstrates that Indian companies can not only produce cost-effective medical solutions but also innovate and compete with established international players in terms of technology and quality.
The successful certification process involved rigorous testing and evaluation by European notified bodies, confirming that the stent meets the highest international standards for medical devices.
As cardiovascular diseases continue to be a leading cause of mortality worldwide, innovations like the Supraflex Cruz stent represent hope for improved patient care and outcomes across the globe, while showcasing India's growing capabilities in advanced medical technology.






