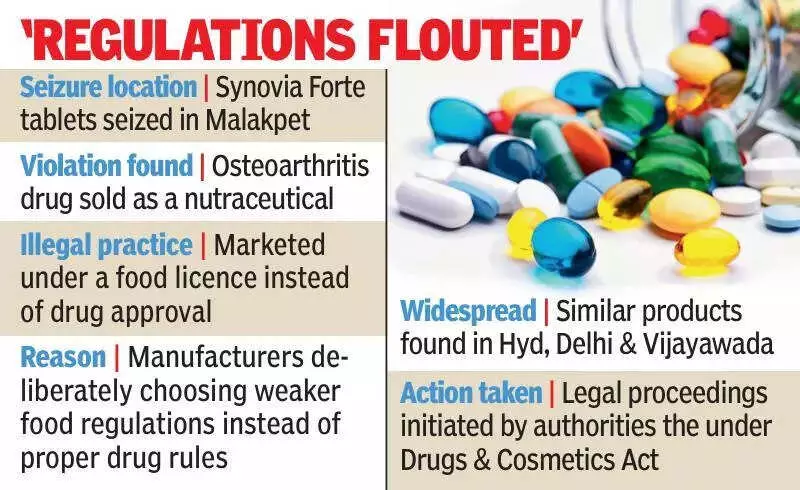
In a major regulatory crackdown, the Telangana Drugs Control Administration (DCA) has uncovered at least nine instances where pharmaceutical manufacturers were producing medicines but deliberately marketing them as 'food products' or 'nutraceuticals'. This deceptive practice was used to evade stringent drug regulations designed to ensure patient safety.
The Statewide Pattern of Misclassification
Over the past few months, DCA officials have detected a consistent pattern across the state. Companies were found to be bypassing mandatory drug manufacturing licences, Good Manufacturing Practices (GMP), quality testing, and regulatory inspections by falsely labelling their products. The main products involved were iron- and zinc-based formulations meant for therapeutic use.
Raids and seizures were conducted at multiple locations. In Uppal, Calcium-D3 tablets were mislabelled as food items. Further actions followed in Musheerabad (Foron-XT tablets), Habsiguda (Ribovin tablets), Mancherial (Prokalk-Z zinc drops), and Malakpet (Dawafer-XT tablets). The products were manufactured and marketed by firms based in both Telangana and Delhi, indicating a coordinated attempt to flout the law.
Key Raids and Seizures
In one significant case, DCA officials raided a pharmaceutical company in Kushaiguda, Kapra, and seized Calgro-D3 tablets. These tablets contained active pharmaceutical ingredients (APIs) that legally require drug approval. In another raid in Ramagundam of Peddapalli district, officials confiscated RedFer-XT tablets, which contained ferrous ascorbate and folic acid.
According to DCA records, these tablets were falsely manufactured and sold under the guise of ‘food products/nutraceuticals’ using only a food licence. This allowed the manufacturers to completely avoid the scrutiny of pharmaceutical regulators. The seized products contained APIs like ferrous ascorbate, folic acid, zinc sulphate, vitamin D3, and calcium—substances with well-established pharmaceutical uses for treating conditions like anaemia, nutrient deficiencies, and bone health issues.
Serious Public Health Implications
A senior DCA official warned that such violations pose a grave threat to public health. The official explained that drug manufacturers are legally bound to follow strict GMP norms, obtain licences, undergo regular inspections, and rigorously test their products for safety and efficacy.
"Food products face far fewer requirements," the official stated. "Standards are weaker, approvals are faster, oversight is limited, and compliance costs are lower. This allows manufacturers to avoid drug inspections and safety testing."
The official emphasized that this practice puts patients at significant risk. Products made outside the strict drug regulatory framework may suffer from poor quality control, incorrect dosages, potential contaminants, or reduced potency, endangering those who consume them believing them to be safe, regulated medicines.
The DCA's investigation reveals a deliberate attempt by certain manufacturers to exploit regulatory loopholes for profit, compromising patient safety across Telangana. The agency is likely to intensify its vigil and take stringent action against such malpractices.





