India's pharmaceutical sector is facing a major regulatory overhaul as the country's top drug authority intensifies quality control measures against small and medium-sized manufacturers. The Drugs Controller General of India (DCGI) has launched a nationwide crackdown to enforce stricter manufacturing standards amid growing concerns over drug safety.
What Triggered the Regulatory Crackdown?
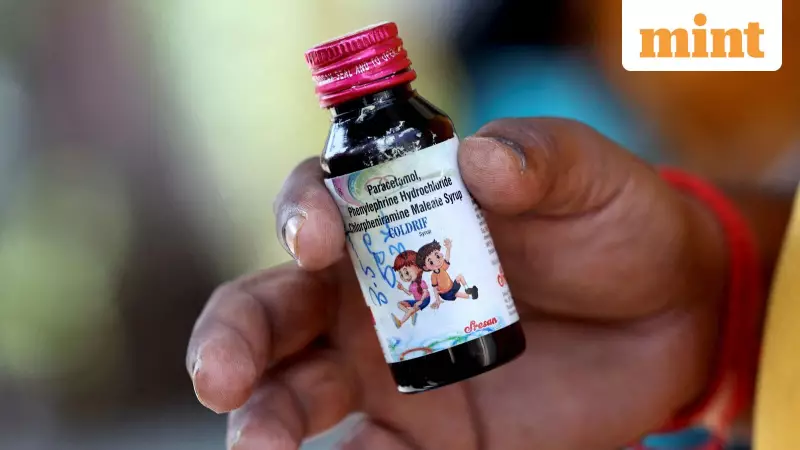
The immediate trigger for this regulatory action comes from recent international alerts about contaminated Indian-made drugs. The World Health Organization flagged three Indian pharmaceutical brands—Coldrif, Respifresh TR, and ReLife—for containing dangerous levels of toxic elements. These cough syrups, manufactured by Sresan Pharmaceutical, Rednex Pharmaceuticals, and Shape Pharma, contained diethylene glycol (DEG), an industrial solvent that can cause serious injury or death, particularly in children.
This isn't the first such incident. The move follows a series of tragic deaths among children in The Gambia and Uzbekistan in 2023 linked to contaminated cough syrups exported from India. More recently, domestic cases in Jammu have further highlighted the urgent need for quality control improvements.
The DCGI's Directive to State Authorities
In a letter dated November 7, 2025, DCGI Rajeev Raghuvanshi directed state drug authorities to conduct immediate inspections of thousands of micro, small, and medium enterprises (MSMEs) that had applied for extensions to comply with revised Schedule M guidelines.
The regulator has set a hard deadline of January 1, 2026, for complete compliance with the upgraded good manufacturing practices (GMP). For MSMEs that didn't apply for extensions, the directive calls for immediate action if they're found non-compliant. States including Delhi, Himachal Pradesh, and Uttarakhand have already begun issuing warning and closure notices to violators.
This crackdown ends the one-year grace period originally granted to MSMEs with annual turnover below ₹250 crore. Interestingly, officials note that very few companies actually applied for the extension despite the challenging requirements.
What Are the New Schedule M Requirements?
The revised Schedule M, upgraded in December 2023, represents a significant leap in India's pharmaceutical quality standards. The new framework aligns with global standards like WHO GMP and requires comprehensive changes across manufacturing operations:
- Implementation of a pharmaceutical quality system for managing quality across all manufacturing aspects
- Computerized storage systems for data management
- Upgraded equipment meeting global quality standards with proper validation
- Complete traceability of supply chains and use of qualified vendors
- Mandatory testing of both finished products and raw materials
- Enhanced facility requirements including air quality monitoring and microbiology labs
- Installation of HVAC systems critical for maintaining sterile environments
Why MSMEs Are Struggling to Comply
India's pharmaceutical landscape comprises over 10,000 manufacturing units, with approximately 80% being MSMEs with turnover below ₹250 crore. These smaller players face multiple challenges in meeting the new standards.
The capital investment required for upgrades often exceeds what these companies can manage through internal reserves, while access to external funding remains difficult. As Harish K. Jain, president of the Federation of Pharmaceutical Entrepreneurs, noted in July 2025, the financial burden is substantial.
Beyond financial constraints, MSMEs struggle with operational changes. Setting up a pharmaceutical quality system requires trained personnel, regular quality reviews, and expensive compliant software systems. The infrastructural changes—including HVAC systems, air quality monitoring tools, and microbiology labs—represent both technical and financial hurdles.
Industry representatives had sought an extension until December 2026, but the health ministry only offered until December 31, 2025, for those who applied with upgraded plans.
The investigation into Sresan Pharmaceuticals' facility revealed shocking violations, including absent pest control, rusty equipment, and use of non-pharmaceutical grade materials. Despite these conditions, the manufacturer retained its license since 2011, exposing significant regulatory gaps that the current crackdown aims to address.