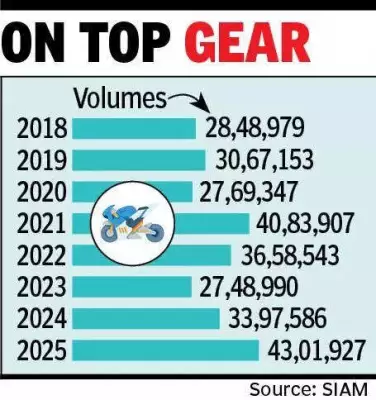
The landmark India-European Union Free Trade Agreement (FTA) is poised to significantly strengthen the position of Indian pharmaceutical formulations and medicines within the European market. This strategic trade pact is expected to catalyze a new era of growth for India's robust pharma sector by addressing key barriers to entry and expansion in one of the world's most regulated markets.
Enhanced Market Access and Competitive Edge
According to Namit Joshi, Chairman of the Pharmaceuticals Export Promotion Council of India (Pharmexcil), the FTA's provisions for reduced tariffs and smoother market entry protocols will directly empower Indian drug manufacturers. These changes are not merely procedural; they represent a fundamental shift in the trade landscape that will enhance the sector's ability to scale exports, invest in compliance, and integrate more deeply into European supply chains.
Key Benefits for the Indian Pharma Industry
The agreement is anticipated to deliver multiple advantages for Indian companies seeking to establish or expand their footprint in Europe:
- Cost Competitiveness: Lower import duties will make Indian generic medicines and formulations more price-competitive against European and other international products.
- Regulatory Harmonization: Streamlined approval processes and mutual recognition agreements will reduce the time and cost associated with bringing products to market.
- Supply Chain Integration: Easier access will facilitate partnerships with European distributors, hospitals, and retail chains, embedding Indian manufacturers into the regional healthcare ecosystem.
- Investment in Quality: The prospect of stable, long-term market access will encourage Indian firms to invest further in upgrading manufacturing facilities to meet stringent EU Good Manufacturing Practice (GMP) standards.
This development comes at a crucial time when global supply chains are being reevaluated. Europe's push for diversified sourcing in critical sectors like healthcare aligns perfectly with India's capabilities as the 'Pharmacy of the World'. The FTA effectively formalizes this partnership, creating a predictable framework for trade that benefits both economies.
The successful implementation of this agreement could serve as a model for future trade deals, further solidifying India's position as a global leader in affordable, high-quality medicine production. Industry analysts suggest that the positive impact will be felt across the value chain, from active pharmaceutical ingredient (API) manufacturers to finished dosage form exporters.