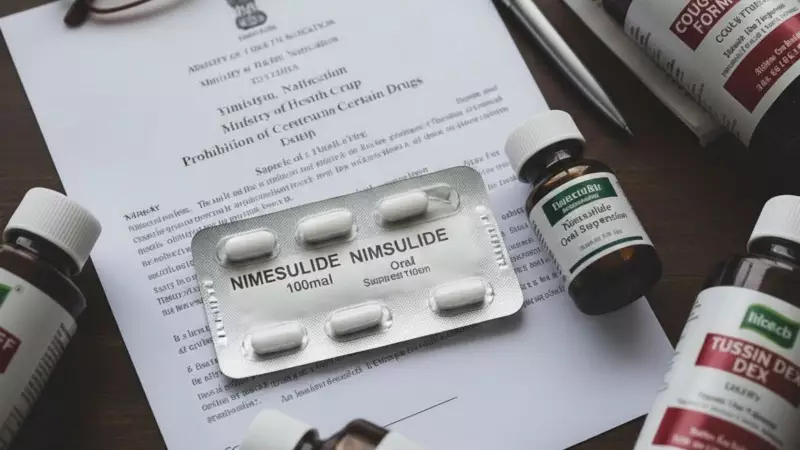
Following a directive from the central government, the state of Haryana has officially prohibited the manufacture, sale, and distribution of high-dose formulations of the common pain and fever drug, Nimesulide. The ban, announced on Thursday, January 1, 2026, specifically targets all oral "immediate release" formulations containing more than 100 mg of the medication.
Government's Firm Stance on Public Safety
Haryana Health Minister Arti Singh Rao stated unequivocally that the government "will not compromise on public health under any circumstances." She emphasized that the action was driven solely by safety concerns. "Medicines are meant to heal, not harm. When scientific evidence highlights potential risks and safer alternatives are available, it becomes the government’s responsibility to act firmly," Rao explained.
The minister confirmed that strict instructions have been issued to ensure full compliance across the supply chain. "Regular inspections and monitoring mechanisms are already in place, and violations will invite stringent action," she warned. Rao also appealed to doctors and healthcare professionals to prescribe safer alternatives in line with medical guidelines and to properly educate their patients.
A Decision Backed by Scientific Review
Contrary to perceptions of a rushed move, a senior Haryana government officer clarified that the decision was "not sudden, and followed a thorough evaluation of safety evidence and expert consultations." The ban comes a day after the Union Government issued a similar notification based on an in-depth review by the Drugs Technical Advisory Board (DTAB).
Lalit Kumar Goyal, the Drugs Controller for Haryana, detailed the rationale. "The Board examined studies and reports indicating that higher-dose immediate-release Nimesulide may increase the risk of adverse effects, particularly when used improperly or for extended periods," Goyal said. He added that such formulations could pose unnecessary health risks when safer therapeutic options exist.
Ensuring State-Wide Enforcement
The central government's notification explicitly required state authorities to enforce the ban strictly. The senior officer noted that states were expected to act promptly to prevent the continued circulation of the prohibited drug formulations. "By promptly enforcing the Centre’s notification, the Haryana government has reaffirmed its commitment to science-based policymaking and safeguarding the health of its citizens," stated Drugs Controller Goyal.
The enforcement directive covers all entities involved in the drug trade:
- Drug manufacturers
- Wholesalers and distributors
- Retail chemists and medical stores
The state's decisive action underscores a coordinated national effort to prioritize patient safety and eliminate potentially harmful high-strength painkillers from the market, ensuring public health remains paramount.