Politics
West Asia Conflict's Political Ripple in Kerala Elections: BJP vs Congress-CPM
BJP's Rajeev Chandrasekhar accuses Congress and CPM of ignoring Malayali Gulf workers and appeasing Iran, as Sonia Gandhi's statement on India's silence sparks debate in poll-bound Kerala.
World
Small Plane Crashes into Hudson River, Pilot and Passenger Escape Safely
A single-engine Cessna 172 crashed into the Hudson River near Newburgh after an emergency landing attempt. The pilot and passenger escaped the wreckage, swam to shore, and were treated for minor injuries. The FAA is investigating the cause.
Business
Middle East Conflict Chokes India's Basmati Rice Exports, 400,000 Tons Stranded
India's basmati rice shipments are severely impacted by the Middle East conflict, with 400,000 metric tons stranded and export deals halted due to doubled freight rates.
Entertainment
Arjun Bijlani Praises Mumbai as a Land of Opportunities and Resilience
Actor Arjun Bijlani shares insights on Mumbai's demanding yet rewarding nature, emphasizing hard work, discipline, and the city's role in personal and professional growth.
Sports
Lifestyle
Technology
OpenAI Loses 1.5M Users After Pentagon AI Deal Backlash
OpenAI faces a major backlash as over 1.5 million users abandon ChatGPT in 48 hours after it allowed the US Department of Defense to use its AI models on classified networks. Rival Anthropic's refusal of similar deals boosts its Claude chatbot.
Dr. Gurtej Sandhu's 2,211 Patents Exceed Thomas Edison's Record
Dr. Gurtej Sandhu, an Indian-born semiconductor inventor, holds 2,211 global patents, surpassing Thomas Edison's 1,432. His innovations power smartphones, cameras, and cloud servers worldwide.
Nothing Phone (4a) in 4 Colors with Periscope Camera
Nothing CEO Carl Pei confirms the Phone (4a) will debut in White, Black, Pink, and Blue, featuring a periscope camera and Snapdragon chipset, with launch on March 5.
Nvidia's AI Chip Empire Faces US Military Ban Threat
Nvidia's neutral stance as AI chip supplier is jeopardized by a US Defense Secretary order banning military contractors from business with Anthropic, potentially forcing Nvidia to cut off one of its biggest customers.
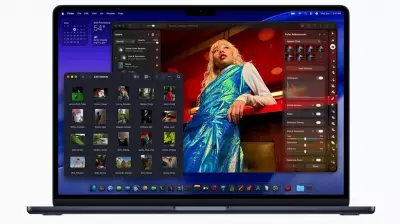
Apple Launches MacBook Air M5 & Studio Display Series
Apple has introduced the new MacBook Air M5 with double the base storage and the Studio Display series, marking significant advancements in its product lineup for 2026.
Health
Get Updates
Subscribe to our newsletter to receive the latest updates in your inbox!
We hate spammers and never send spam